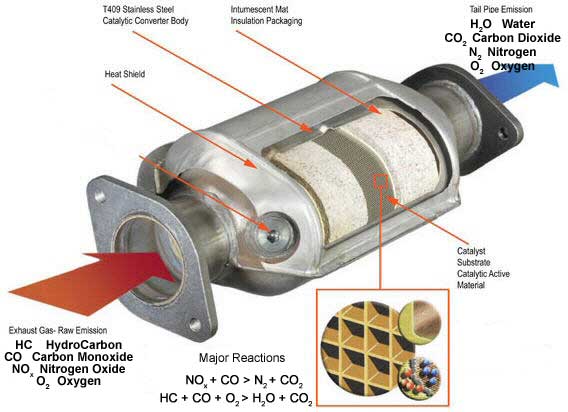
No matter how well an engine runs there will always be harmful pollutants present in the exhaust. These pollutants are by products of combustion and as pollution regulations became stricter, a means of reducing these toxic pollutants was required. The catalytic converter does this job extremely well. A catalytic converter is a muffler looking device located towards the front of the exhaust system of all modern motor vehicles. The converter is always located to the front of the vehicles as close as practicable to the engine exhaust outlet. The converter is easily identified by the addition of heat shields around the device. By using a three-way catalytic converter in the exhaust system, more than 90% of the three most toxic pollutants, Carbon Monoxide (CO), HydroCarbons (HC) and Nitrogen Oxides (NOx) can be degraded or chemically changed at the same time to harmless substances like Carbon Dioxide (CO2), Nitrogen (N2)and water vapour (H2O).
Some of the first catalytic converters contained catalyst coated pellets packed into the converter housing. This first design was a little restrictive and created more exhaust back pressure than the later monolith design. The monolith converter shell contains a ceramic "honeycomb" which is coated with a precious metal, Platinum/Palladium (oxidising catalysts for HC and CO), Rhodium (reducing catalyst for NOx) and Cerium (promotes oxygen storage to improve
oxidation efficiency). When the exhaust gas flows through this honeycomb, these precious metals accelerate the chemical degradation of the toxic pollutants CO, HC and NOx. The converter starts to do its job with the exhaust temperature at 280oC but for maximum converter efficiency the exhaust temperature should be at least 400oC. This is the reason that the converter is placed as far forward in the exhaust as practicable. Efficiency of the converter is also greatly affected by
the initial exhaust composition as it exits the engine. In theory the exhaust composition changes rapidly between high CO and high O2. This continuous rapid fluctuation from rich to lean promotes the conversion rate of the converter. This is where the importance of the O2 sensor is under rated. When the sensor reaction time is diminished, so to is the efficiency rate of the catalytic converter and fuel economy also suffers as a result. The O2 sensor plays a vital role in the engine
management system and the efficient operation of the catalytic converter. Other factors that will affect the life and efficency of a catalytic converter is the condition of the engine, ignition system, fuel system, and all emission devices. All these components must operate within manufacturer specifications, and correctly at all times.
The engine must burn close to the optimum fuel mixture with an excess air factor of Lambda = 1.00 and must be maintained within a very small band of deviation from this figure otherwise the converter cannot operate efficiently. The only way to control this figure is to use an extremely accurate closed-loop control, featuring almost zero lag, to the air fuel mixture management system. This is where the O2
sensor comes into play. Also any engine miss-firing and/or excessive un-burnt fuel reaching the converter will raise the exhaust temperature beyond the substrate fusion point and subsequently the substrate will melt the ceramic monolithic structure. When this happens, obviously the catalytic converter is destroyed, this also will cause a major restriction for the exhaust gas with the consequences of reduced power and excessive fuel consumption. From my experience I have noticed that the catalytic converter is
rarely replaced on its own from a diagnosed fault. The are often overlooked and only replaced when the rest of the exhaust system goes rotten. Unfortunately the other reason they are overlooked is because of the high cost of replacement. Sometimes they are totally removed and in my opinion this option should never be contemplated. They are a consequence of going green and if you support the green movement then you won't mind spending the money to replace them as necessary. In summary, I can only say that to get
the maximum life from your catalytic converter means regular servicing and tune-up at the recommended intervals. If you notice the engine running poorly, have it fixed immediately. The converter will fail fairly quickly if the engine is left in a poor state of tune.
Click this link to see how to test a Catalytic Converter